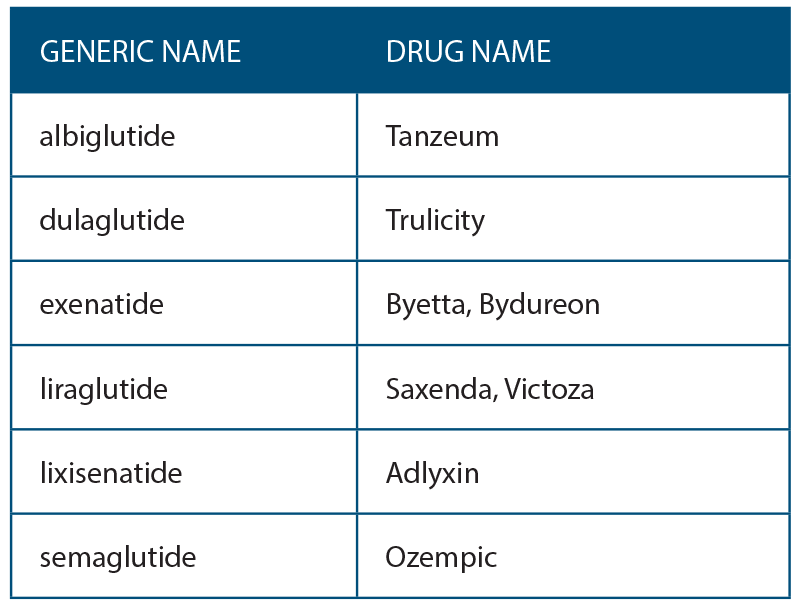
A new class of medications for the treatment of type 2 diabetes mellitus (DM) called glucagon-like peptide 1 receptor agonists (GLP-1R) has been available since 2005. They are part of a larger group of incretin mimetics. Several marketed drugs that are FDA approved are currently included in this group (See Table 1, right). These drugs are injectable and not recommended as primary treatment for DM. They are indicated for patients with hyperglycemia or elevated hemoglobin A1c despite being on oral antihyperglycemic medications.
All of these drugs carry a warning regarding the possible association with medullary thyroid cancer (MTC) or multiple endocrine neoplasia syndrome 2 (MEN2). In rodents, liraglutide activated GLP-1 receptors on rat thyroid C-cells and has caused tumors in experimental animals (Endocrinology 2010;151:1473–1486). Currently there is no evidence that it causes medullary thyroid cancer or affects MEN2 in humans (Exp Diabetes Res. 2012; 2012:924168; J Clin Endocrinol Metab 2011; 96:853–860). According to the websites of these drugs, there are warnings that there may be dose-dependent and treatment duration-dependent risk for C cell tumors at “clinically relevant exposures.” This has not been studied in humans and, thus far, there are no reports of these findings.
Discussion
GLP-1 receptors have been found on follicularly derived papillary thyroid cancer cells (Diabet Med. 2018; 35:381–385). These receptors were not found in normal follicular cells. Again, the significance of this is unclear but would warrant prolonged observation of diabetic patients on GLP-1 receptor agonists. A review of the FDA adverse event database revealed an increased incidence of thyroid cancer with exenatide whn compared with a control drug (J Clin Endocrinol Metabolism. 2012; 97:121–131).
Although the evidence for the development of human MTC is lacking, this class of drugs has been contraindicated in patients with medullary thyroid cancer, a family history of medullary thyroid cancer, or associations with MEN 2 syndrome. The warnings also recommend routine monitoring of serum calcitonin and thyroid ultrasonography while on these drugs. They also state that obtaining these tests is of uncertain value. A recent case report of a woman who was diagnosed with MTC while on a GLP-1 agonist showed no change in her calcitonin levels (Gastroenterology. 2011;141:150–156).
The incidence of MTC in the United States is 0.22/100,000. The prevalence is estimated to be 1/14,300 (orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=1332). With the advent of these new drugs, it is likely that our specialty will see these patients and they may ask why there is this risk. Patients with newly diagnosed thyroid or neck masses should be asked about the use of these medications. Currently, the FDA is advising against the use of GLP1 agonists in people at risk for MTC or MEN2 syndrome (N Engl J Med. 2010;362:774–777).
Obtaining a history from a patient with a newly diagnosed thyroid or neck mass should include a history of taking GLP-1 drugs.
Implications for Practice
While it is unlikely that otolaryngologists will be prescribing GLP-1 medications, it is likely that they will see patients using these drugs or be referred patients who develop neck masses. Obtaining a history from a patient with a newly diagnosed thyroid or neck mass should include a history of taking GLP-1 drugs. Our patients with MTC or those who are family members of patients with MTC should not be taking these medications at this time due to the risk. An awareness and knowledge of this adverse effect are necessary.
David Myssiorek, MD
Department of Otolaryngology Head and Neck Surgery
Bronxcare Health System
Bronx, NY
Vivien Leung, MD
Department of Endocrinology
Bronxcare Health System
Bronx, NY