The terms “protocol” and “practice parameter” are often used interchangeably with “clinical guideline,” although these types of evidence do not always match the methodologic rigor required of guidelines as specified by criteria established in the Appraisal of Guidelines Research and Evaluation instrument widely used to measure guideline quality (Otolaryngol Head Neck Surg. 2009;140(6 Suppl 1):S1–43). An understanding of how clinical guidelines are developed may help clarify how these guidelines differ from other types of evidence.
Explore This Issue
July 2012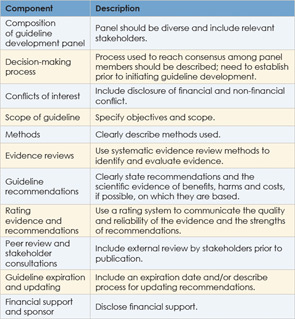
Developing Clinical Guidelines
A number of criteria have been proposed to develop clinical guidelines. A study published in 2008 identified key aspects commonly used among the various approaches (Implement Sci. 2008;3:45). These include: identifying clinical questions or problems; systematically reviewing and appraising the literature; drafting recommendations; external consultation and review; and a planned update. This process should be done by a multidisciplinary panel with input from consumers. In addition, a number of criteria designed to assess the quality of clinical guidelines have been proposed. The Appraisal of Guidelines for Research and Evaluation and the Conference on Guideline Standardization are two commonly used criteria.
Another set of criteria commonly used by guideline developers has been established by one of the main organizations disseminating clinical guidelines. The National Guideline Clearinghouse (NGC), a publicly available database of evidence-based clinical practice guidelines formed in late 1998 under the Agency for Healthcare Research and Quality (AHRQ) in the U.S. Department of Health and Human Services, currently disseminates about 2,700 guidelines. All guidelines published through the NGC must meet its criteria.
Even with the plethora of criteria available, many guidelines do not meet quality standards. A 2011 report by the Institute of Medicine (IOM) attributed this problem to shortcomings in the development of guidelines and proposed its own criteria to ensure trustworthiness. The IOM recommends that high-quality clinical guidelines:
- Be based on systematic review of the existing evidence;
- Be developed by a knowledgeable, multidisciplinary panel of experts and representatives from key affected groups;
- Consider important patient subgroups and preferences;
- Be based on explicit and transparent processes that minimize distortions, biases and conflicts of interest;
- Provide a clear explanation of the logical relationships between alternative care options and health outcomes and rate both the quality of evidence and the strength of the recommendations; and
- Be revised as appropriate when new evidence warrants modifications of recommendations.
The IOM also proposed standards for developing trustworthy guidelines. It urges attention to:
Leave a Reply