Results from Europe indicate that biosimilars are generally very safe. “In Europe, patients are switching from a branded biologic directly to a biosimilar and maintaining the same level of disease control without developing safety issues,” Hakim said.
Explore This Issue
August 2017What Otolaryngologists Need to Know
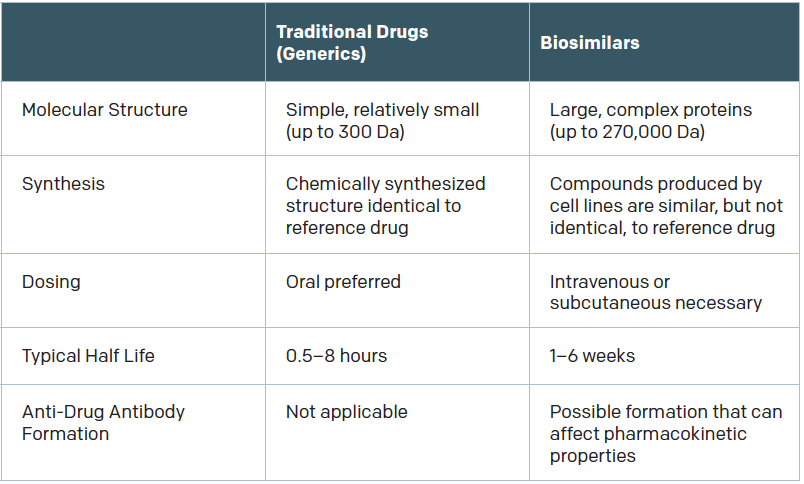
Biologics are relatively new within the field of otolaryngology, and, so far, no biosimilars have been FDA approved for otolaryngologic indications. But given the emergence and promise of biologics in the treatment of allergy, asthma, and chronic rhinosinusitis, that may change.
Omalizumab, mepolizumab, and reslizumab are FDA approved for asthma, omalizumab is used to treat severe allergy-related asthma, and mepolizumab and reslizumab are approved for eosinophilic asthma. Benralizumab, an anti-IL-5 receptor alpha antibody, is currently being investigated as a treatment for severe asthma, and dupilumab, an interleukin-4 receptor alpha antagonist, is being evaluated for atopic dermatitis. Nemolizumab, an investigational humanized monoclonal antibody against interleukin-31, is also being investigated for resistant atopic dermatitis.
In the near future, biosimilar use in otolaryngology will likely occur primarily in the areas of allergy and immunology. In Europe, seven of the 31 products that received a positive opinion from the EMA Committee for Medicinal Products for Human Use have an indication for allergic and immune diseases (J Allergy Clin Immunol. 2017;139:1461-1464).
Biologics and biosimilars may also play a role in the treatment of chronic rhinosinusitis. “There are a fair number of biologics that are being tested for chronic rhinosinusitis,” said Stacey Gray, MD, assistant professor of otolaryngology at Harvard Medical School in Boston. “The idea is that, hopefully, this will be helpful for our patients with really recalcitrant chronic rhinosinusitis, but we’re not there yet.” Some small studies have shown potential benefit, but “a lot of the data has not actually been published yet,” Dr. Gray added. Larger, randomized-controlled trials will be necessary to determine the efficacy of biologic—and perhaps biosimilar—treatment for rhinosinusitis.
Interchangeability Is Important
For biosimilars to take off in the U.S., experts say it’s likely that drug manufacturers will have to demonstrate—and the FDA will have to confirm—interchangeability between biosimilars and their reference biologics. (To date, the biosimilars approved in the U.S. have been deemed “highly similar.”)
Interchangeability will give both consumers and clinicians the confidence that one product is as effective as the other, and that confidence is necessary to convince people to switch from expensive biologics to likely cheaper biosimilars. In fact, until patients and clinicians are willing to switch to biosimilars, cost savings might not materialize as hoped.