Opioid use following otolaryngology surgical procedures has received increased interest, with many studies recommending shorter-term prescriptions for opioids to avoid dependence on the drugs. In response to a continuing opioid […]
FDA Recalls Zantac
Updated April 10, 2020. The U.S. Food and Drug Administration announced on April 1 that it is immediately pulling all prescription and over-the-counter ranitidine (brand name Zantac) drugs from the […]
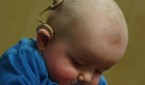
Off-Label Cochlear Implantation Common
A new study concludes that cochlear implantation is commonly performed for indications beyond those included in FDA-approved labeling
FDA Works to Increase Consumer Access to Hearing Aids
The FDA takes steps to better support consumer access to hearing aids.

Reducing Costs in the Changing Fabric of Healthcare
Lecturer describes shift in healthcare from 1946 through the ACA

How the U.S. FDA Approves Medical Devices
Outline of steps in the federal approval route, from idea phase to implementation
FDA’s Unique Identifier Program Has Benefits, Drawbacks
The proposed rule may assist with safety, but be costly.
FDA Seeks to Prevent Surgical Fires
While surgical fires are exceedingly rare they can have devastating consequences. The FDA and a coalition of health care providers recently launched the Preventing Surgical Fires Initiative to help physicians manage the risk of surgical fire.