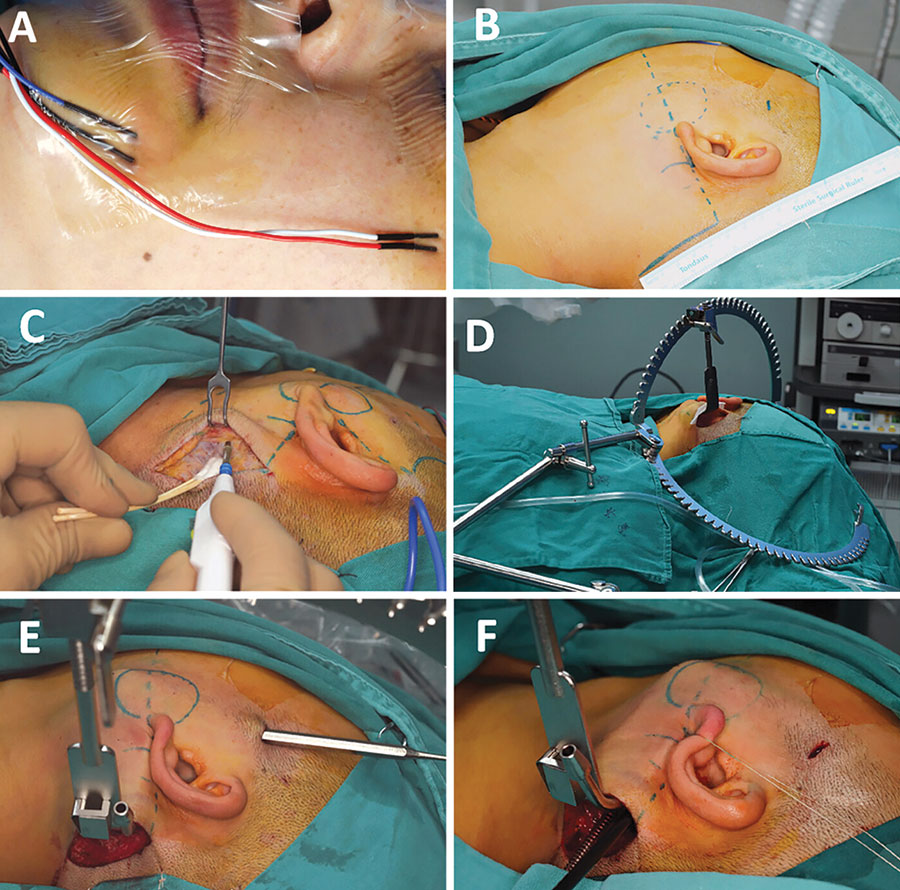
Figure 2: Pre-operative preparation and the creation of surgical cavity under direct view. (A) The placement of terminals of neuromonitor. (B) The design of incisions and working cavity. (C) Incise the skin under direct view. (D) The use of surgical retractor systems. (E) Fusion of two surgical cavities. (F) Pull the earlobe to enlarge the surgical cavity.
Surgical separation began with the posterior boundary of the parotid, defined by the anterior tissue of the sternocleidomastoid muscle. Following adequate exposure of the superficial parotid tissue, the retromandibular vein became visible in the deep region of the neoplasm. An ultrasonic scalpel and vascular clap were used to ligate and cut the distal end of the retromandibular vein. Subsequent antegrade dissection allowed clear identification of the facial nerve trunk and its branches as the superficial parotid gland was elevated by the “plus” instrument. The electromyographic signal was collected by placing the neuromonitor on the branches of the facial nerve, which may help to identify the nerve tissue and reduce the risk of facial palsy. After the proximal end of the retromandibular vein and adjacent tissues were cut, the neoplasm and superficial parotid gland were completely excised and sent for rapid frozen pathological examination.
Explore This Issue
May 2025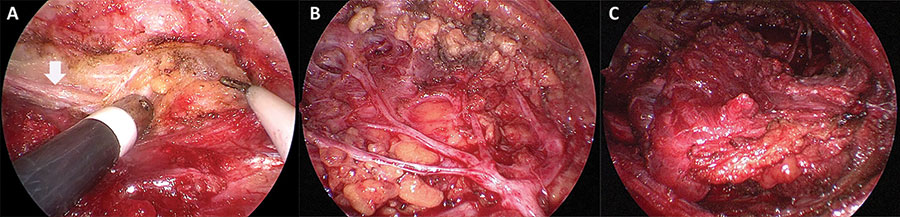
Figure 3: Magnified structures under endoscopic view. (A) The great auricular nerve was identified when creating surgical cavity (vertical arrow). (B) The trunk and branches of facial nerve were exposed following the excision of superficial lobe of the parotid. (C) The sternocleidomastoid muscle flap was transferred to cover the facial nerve.
The intra-operative pathological report indicated mucoepidermoid carcinoma, which required total parotidectomy to ensure a tumor-free margin. Consequently, we continued to excise the remaining deep lobe tissues of the parotid using an ultrasonic scalpel or surgical scissors. Due to the advantages of magnification and illumination of the endoscope, the anatomic structures in the surgical field were identified clearly after irrigation with normal saline, facilitating the effective conservation of facial nerves and branches (Fig. 3B). Following the removal of deep lobe tissues, neural integrity was reassessed by observing the waveform on the neuromonitoring device. In the final step, the local SCMF was designed and transferred as a reconstruction modality. The local flap was divided from the anterior border of the SCM, with a thickness of up to one-third or half of the muscle. Careful attention should be given when dissecting the muscle to avoid injury to the accessory nerve. The flap was then rotated forward to fill the defect and cover the surface of the facial nerve, and it was sutured to the surrounding tissues (Fig. 3C).
After transferring the SCMF, the surgical cavity was irrigated through both the postauricular and the “plus” incisions. Local bleeding spots were clearly visible under the endoscopic view, and hemostasis was achieved with long bipolar electrocoagulation. The incisions were closed in layers following the placement of a negative-pressure drainage tube. Post-operatively, a bandage was applied to the parotid area with appropriate pressure.
RESULTS
The operation was successfully completed without converting to conventional open surgery. The incisions were hidden in postauricular and temporal regions, with lengths of 5.5 and 1 cm, respectively. The total operation duration was 230 minutes, of which 195 minutes were completed under endoscopic vision. The final pathological diagnosis was well-differentiated mucoepidermoid carcinoma (pT2N0M0).
Post-operatively, the patient remained hospitalized for five days and was discharged as planned. Temporary numbness of the auricular region was identified, resolving gradually over three weeks. At a 32-month follow-up, no severe complications or recurrence were observed. The patient reported high satisfaction with post-operative facial function and aesthetic outcomes.
CONCLUSION
FETP combined with SCMF transplantation could achieve excellent aesthetic outcomes with minimal complications, and it is a safe and feasible option for patients diagnosed with low-grade malignant parotid tumors.
Leave a Reply