Outcomes research seeks to expand upon studies of clinical efficacy, which generally measure the biological effects of an intervention under ideal circumstances in a rigidly defined and controlled group of patients. Outcomes research is concerned with effectiveness, or the impact of the intervention on the overall health and well-being of the typical patient under real-world circumstances. Although the scope of most efficacy studies is necessarily narrow-constrained by the need to maintain control over experimental variables-the scope of outcomes research is broad. For example, whereas efficacy studies would limit a research question to the impact of an intervention on certain biological markers, outcomes research would consider the impact of that same intervention on overall patient health, including physical, social and vocational functioning, quality of life, pain control, symptom relief, emotional status, and satisfaction with care-the parameters that patients typically care about.
Explore This Issue
January 2008Why Measure Patient Outcomes?
There have never been more reasons to measure patient outcomes. The changing health care marketplace is demanding new levels of accountability from providers, health care systems, and health plans. Purchasers and patients want evidence that health care dollars are used for high-quality, effective care-treatment that will improve a person’s health and daily functioning as a whole human being, not just a simple biological system. Patients judge the effectiveness of a drug or procedure by its impact on their lives; they want to know whether the treatment will relieve their most significant symptoms and in the process alleviate anxiety, depression, and frustration. Clinicians need to have better insight into the impact of various management approaches on patients’ long-term clinical status and quality of life. Building a database of patient experiences across treatments and conditions can help provide this type of information-and can help the specialty, future patients, and the individual clinician.
Types of Outcomes Instruments
Health-related quality-of-life outcome measures encompass physical and social functioning, activities of daily living, bodily pain, health perception, and mental health. These measures may be either generic, disease-specific, or intervention-related. Generic health status measures are broadly applicable across practice settings, health conditions, and medical treatments, but are not particularly sensitive to many common conditions in otolaryngology such as mild to moderate hearing impairment, voice disorders, and rhinosinusitis. Some well-known and widely-used generic health status measures include the Medical Outcome Study Short-Form Health Survey (SF-36),2 the Sickness Impact Profile (SIP),3 and the Health Status Questionnaire (HSQ).4
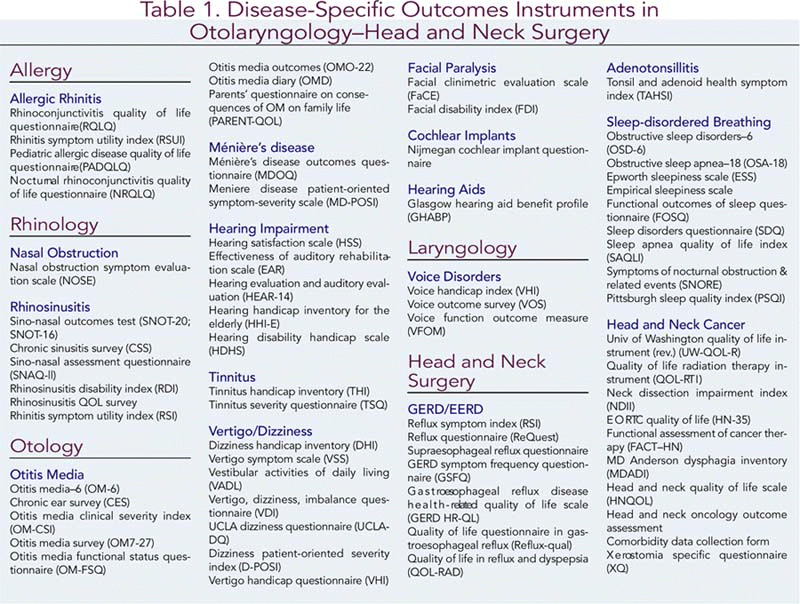
Disease-specific and intervention-related outcome measures have proliferated over the past decade as they are able to capture more detailed data regarding function affected by the underlying condition and make the attribution of clinical change to treatment more straightforward.5 Table 1 lists 68 disease- and intervention-specific outcome measures in otolaryngology-head and neck surgery. Although this list is not comprehensive, many of the common diseases, disorders, and conditions treated by otolaryngologists are represented in the list, offering a choice of instruments for some conditions. All are patient-completed forms (rather than requiring responses through a personal or telephone interview), so that they can be given to your patients for completion prior to the appointment or while in the waiting area of your office. While having the obvious benefit of saving staff time, questionnaires are also less expensive, more uniform, increase patient privacy, and may also increase valid responses when compared to an interview, where patients may feel pressure to give an acceptable response.
How to Choose an Outcomes Instrument
The first criterion for choosing an outcomes instrument for assessment of patients in your practice is what the objective of your study is. What do you want to know-symptom severity and their impact on the patient’s life? The psychological impact of the disorder on the patient and his or her family? The effect of the disorder on activities of daily living? Look for a questionnaire that specifically targets these areas. A number of instruments are divided into domains-such as physical domain, social domain, emotional domain-that are the foci of groups of questions. You will also want to use an instrument that has been validated-that it measures what it purports to measure in the population of interest, that it can differentiate between groups with and without the condition of interest, and that it correlates with well-accepted existing measures of the same condition. You will certainly want an instrument that is reliable-one that can produce consistent results, and consistent results on different occasions, when there is no evidence of change.6 Finally, the ideal instrument will be responsive-it will be able to detect change over time, an important attribute if you wish to study treatment effects or the natural history of a disease. You may also wish to consider the burden of an assessment instrument, both for the patient and for staff members responsible for scoring the results: how long is it and how complicated; does it require special instructions in order to complete; is it available in languages other than English?
Leave a Reply