INTRODUCTION
Parotid tumors (PTs) account for approximately 64% to 80% of salivary gland tumors, with the majority being benign and located in the superficial lobe. Generally, conservative surgical resection is the primary treatment for benign PTs, while total parotidectomy is recommended for malignant parotid lesions to obtain safe histological margins. Following the total parotidectomy, the sternocleidomastoid muscle flap (SCMF) can be used to prevent Frey’s syndrome and partially restore facial contour defects.
Explore This Issue
May 2025Conventional total parotidectomy is typically conducted through a Blair incision, which can leave a visible facial scar, impacting the cosmetic satisfaction and quality of life of some patients. Recently, endoscopic surgery has been increasingly applied to head and neck surgery to achieve minimally invasive effects and improved aesthetic outcomes. There are few reports on the full endoscopic technique of total parotidectomy followed by SCMF transplantation to our knowledge, however.
We aim to describe the surgical procedures of full endoscopic total parotidectomy (FETP) and assess patient outcomes to evaluate the feasibility and efficacy of this novel approach.
MATERIALS AND METHODS
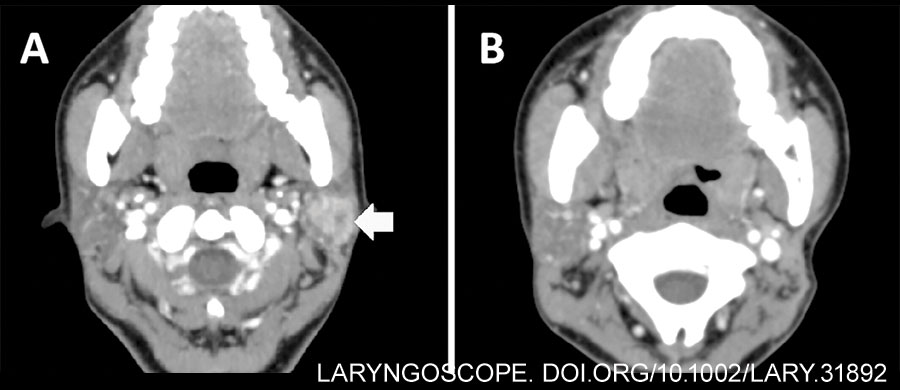
A case of parotid malignancy managed with FETP and SCMF transplantation at our department was retrospectively reviewed. Pre-operative contrast-enhanced CT revealed a 2×2 cm-sized mass with heterogeneous enhancement in the left superficial parotid gland (Fig. 1A). Preparation of hair removal involved a 2-3 cm area in the temporal region above the hairline and the postauricular region behind the hairline.
The operation was conducted according to the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of West China Hospital of Stomatology, Sichuan University (Approval No. WCHSIRB-D-2020-311-R1). The patient was fully informed about the benefits and drawbacks of this procedure, as well as risks of conversion to conventional open surgery.
Surgical Technique
Following general endotracheal anesthesia and routine disinfection, the terminals of the neuromonitor were placed into the subcutaneous tissue of the manubriosternal region, orbicularis oculi, and orbicularis oris, respectively, for intra-operative facial nerve monitoring (Fig. 2A). To improve the post-operative aesthetic results, an approximately 5-cm incision was designed along the postauricular hairline region, with the reflection of neoplasm marked (Fig. 2B). Noticeably, a 1-cm temporal incision and cavity were designed to accommodate the suction device or other endoscopic instruments, facilitating cavity separation, blood suction, and tissue lifting. We referred to these designs as the “plus” incision and cavity in FETP surgery. The electrocautery, sterile cotton swab, and skin retractor were used when incising the skin and subcutaneous tissue (Fig. 2C). Following the creation of the main working cavity, a surgical retractor system was used to lift the skin flap stably and maintain a wide operative cavity (Fig. 2D). Then we expanded the main working cavity using a 5-mm laparoscope with 0° viewing angles and miniature instruments. Manipulation at this step may be obstructed by the cartilage of the external auditory canal. The “plus” cavity can then be established with blunt dissection to proceed with the separation of the superficial musculoaponeurotic system and achieve a distinct anatomical layer (Fig. 2E). Incise the connected fascia under endoscopic view until two cavities are fully merged. Additionally, stitches can be used to pull the earlobe area, achieving a larger operative cavity (Fig. 2F). To reduce post-operative numbness in the auricular skin, careful protection of the great auricular nerve (GAN) is crucial during the extension of the surgical tunnel (Fig. 3A).
Leave a Reply