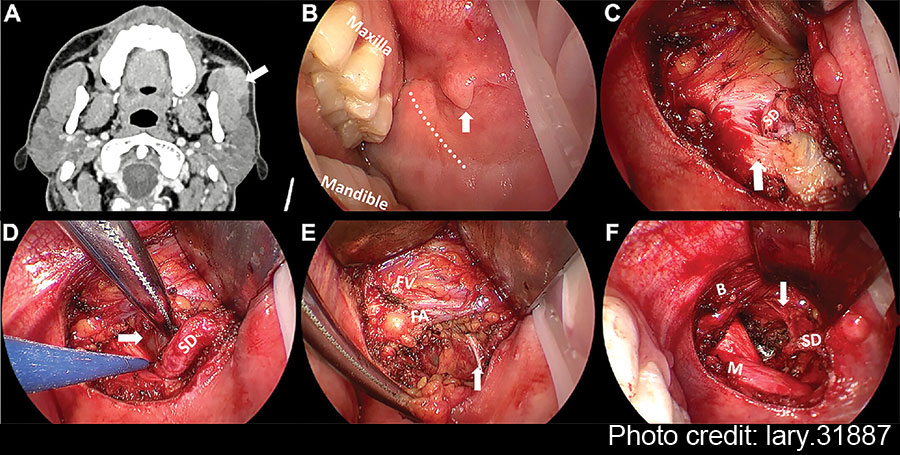
Figure 1: Pre-operative CT and intra-operative images under endoscopic view. (A) The axial contrast-enhanced CT of left APG mass. (B) Pay attention to SD papilla when designing the incision (vertical arrow). (C) The relationship between SD and anterior masseter muscle (vertical arrow). (D) The identification of APG tumor (horizontal arrow). (E) The exposure and protection of buccal branch of the facial nerves (vertical arrow). (F) The surgical bed after the removal of APG tumor. The branch of the facial nerves was identified clearly under magnified view (vertical arrow). APG = accessory parotid gland; B = buccinator muscle; FA = facial artery; FV = facial vein; M = masseter muscle; SD = Stensen’s duct.
Thyroid retractors were then replaced by deep surgical retractors to obtain a wider view of the oral cavity for easier manipulation. Blunt dissection was performed along the tumor capsule and SD posteriorly to separate the surrounding tissue. The APG, buccal fat pad, and part of the parotid were excised together with the mass. At this step, the endoscopic view allowed for clear visualization of the branches of the facial nerves, facilitating the separation and protection (Fig. 1E). In addition, a long bipolar electrocoagulation was recommended for tissue separation due to its precise coagulation capabilities, minimizing damage to adjacent critical tissue and reducing bleeding.
Explore This Issue
April 2025Following the removal of the APG tumor and part of the surrounding tissue without any macroscopic rupture, the surgical field was irrigated with saline solution. The SD and buccal branch of the facial nerves were preserved completely (Fig. 1F). The intra-operative frozen pathology confirmed the diagnosis of pleomorphic adenoma; thus, the conventional parotidectomy was not conducted. Finally, the oral wound was closed with 4–0 absorbable sutures with a rubber drainage strip inserted, which would be removed two days after surgery. The macroscopic operative field and related anatomical structures surrounding the APG tumor were presented in the illustration (Fig. 2). To prevent a salivary fistula, a bandage was applied to the parotid area for approximately one week.
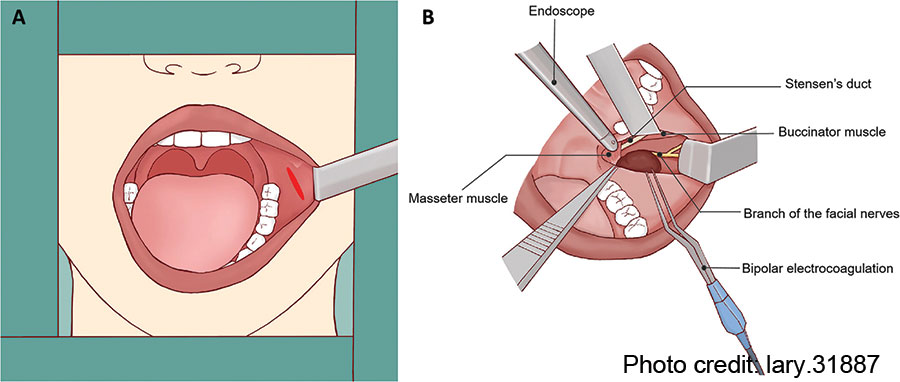
Figure 2: Illustration of the operative field and related anatomical structures. (A) Using surgical retractor to obtain adequate exposure for transoral resection of APG tumor. (B) Anatomical landmarks surrounding the APG tumor.
RESULTS
The procedure was completed within 85 minutes, without conversion from the endoscope-assisted transoral approach to the conventional parotidectomy via Blair incision. Post-operatively, there were no visible facial external scars left, leading to brilliant aesthetic outcomes. The patient used a soft assisted feeding tube to consume a liquid diet six hours after the surgery. The final immunohistochemical report revealed pleomorphic adenoma, consistent with both the pre-operative diagnosis and frozen pathological report.
The patient remained hospitalized for two days post-operatively and was discharged as scheduled. There were no reported complications such as facial paralysis, wound infection, or bleeding issues observed at a follow-up period of six months. The patient reported high satisfaction with post-operative cosmetic outcomes and facial nerve activity.
CONCLUSION
Our reported experience outlines the procedure and tips for endoscope-assisted transoral APG tumor resection. Future prospective multicenter studies with large sample sizes and longer follow-up duration are necessary to further assess the safety and clinical feasibility of this modality. In summary, for suitable benign cases, the endoscope-assisted transoral excision of APG tumors deserves to be further considered in clinical practice.
Leave a Reply